Light-Emitting Diodes (LEDs): Working Principles and Applications
Few parts are as ubiquitous as light-emitting diodes: the lack of classic light bulbs means they get pressed into usage as indicators, even in basic electronics tutorials. This article looks at them in more detail and focuses on less common applications and pitfalls in LED usage.
Physics: How LEDs work and how their spectra look
Even though this text focuses on practical applications, a small excursus into semiconductor physics is justified. A light-emitting diode is a diode that is doted with specific chemicals. These ensure that the excess energy in forward conduction is – partially – emitted as visible or infrared / UV light. Depending on the chemical used for doting, different colors are achieved. Unlike a regular diode, however, LEDs are not well suited to rectification applications – their forward voltage is high, while the reverse breakdown occurs very early.
Initially, manufacturers provided red, green, and yellow LEDs. Blue LEDs have been available for about ten years, with pink and purple LEDs being relatively new constructions (see, e.g., the unboxing video at https://www.youtube.com/watch?v=tFjaeYsQ2vs). Sadly, purple LEDs sometimes yield rather unsatisfactory (read: blueish) colors.
Source: https://www.youtube.com/watch?v=tFjaeYsQ2vs
One crucial takeaway concerns the construction of white LEDs. They are usually constructed by placing a blue LED behind a filter or combining red, blue, and green LEDs to use additive color mixing. Filtering is usually cheaper, while additive mixing yields a "better" spectral composition (see also https://www.sciencedirect.com/science/article/pii/S163107051830029X).
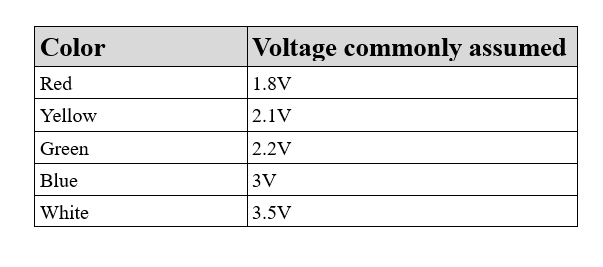
LEDs for signaling
Customer demands usually drive signaling: Clients often demand multiple colors to make signal reception easier for non-technical staff (think of ground chiefs in bus companies, for example). In this case, direct driving of the LEDs from the signal source can keep BOM costs down by eliminating the need for an amplification transistor. In this scenario, however, the ever-lowering signal voltage becomes an issue, especially with the breakdown voltages shown above. Circuits such as the BG96 one shown in the figure work with 1.8V.
Some modern LED chemistries can light up visibly at much lower voltages. Harvatek de Shenzhen produces the product line shown in the figure; the diodes are particularly well suited to this task. When driving LEDs directly from data lines, remember that their high reactivity allows data exfiltration via an optocoupler. This is a specific issue for defense / high-security applications and is mentioned here to sensibilize the reader to this problem. A capacitor in parallel with the LED suffices to satisfy regulatory demands in most cases.
Excursus: Smart LEDs
If many LEDs are to be driven, matrix approaches such as Charlieplexing quickly reach their limits. LED drivers provide a more efficient solution. The modern semiconductor market provides thousands of alternatives with various bus interfaces. We can only mention them in passing here due to space concerns to enable sensibilization. For comparison's sake, the two figures below show WS2812B daisy chain smart LEDs and an LED1202-driven RGB LED bar on a schematic level. The WS2812B system scales up quickly, but the LED1202 system uses a standardized bus and does not require bit banging.
LEDs for lighting plants and humans
Light-emitting diodes usually have extremely narrow spectra; natural light consumers (humans and plants) have different spectral demands. In the case of plant illumination (commonly called horticulture), the biggest issue involves matching the spectral sensitivity of the plant to that of the LED. Most average LEDs are not well suited to that, as shown in the figure below from Wuerths ANO002.
Multiple LED vendors provide so-called horticulture LEDs. These use special chemical doting to ensure that the spectral range matches the plant chlorophyll's: no energy is wasted, which also keeps cooling costs down. Humans add additional difficulty to the process. Sunlight and incandescent bulb emissions tend to have a broad spectrum, which is best accepted by the eye. Furthermore, modifying the light spectrum can promote wakefulness or drowsiness. These topics are covered in some detail at https://www.digikey.hu/en/articles/controlling-tunable-white-leds-for-human-centric-lighting and https://www.ledrise.eu/blog/color-temperature-explained-lr/ – in some cases, combining warm and cool LEDs with a sophisticated dimmer circuit leads to better light bio-compatibility. Finally, driving large amounts of LEDs requires attention to circuit topology. One efficient way involves batching the LEDs up in series and driving them via a constant current source – this is common in LED light bulbs such as the OSRAM G9 3,8W shown in the figure.
Source: https://www.richis-lab.de/LED_02.htm
LEDs for communication
Some LED types achieve border frequencies in the range of multiple hundred Megahertz. The availability of fast photodetectors and robust modulation schemes means visible light can also be used for data exchange. Given that light propagates without a guiding medium, creating VLC communication often requires no modification to existing infrastructure. Furthermore, light travels quite far underwater – if a fleet of submarines is to coordinate, visible light communication can permit high data ranges with comparatively low power expenditure. Sadly, space concerns prevent a more detailed discussion of VLC. However, Peter Adam Hoeher's book of the same name, published by Hanser, provides an excellent overview and is recommended for everyone working in the field.
LEDs as optical sensors
Finally, a short mention of the MIMS effect is required. A light-emitting diode shows a – admittedly weak – response to incoming light. An example is the oscillogram shown in the figure, which was captured (and ERES enhanced;
see
https://cdn.teledynelecroy.com/files/appnotes/an_006a.pdf) with a LeCroy oscilloscope.
LED bulbs cause the waveform in question – their switch mode power supply generates characteristic noise, which is picked up by the light-emitting diode when connected across a scope probe.